Bispecific Antibodies & BiTE® (Bispecific T-Cell Engager)
Clinical & Therapeutic Research
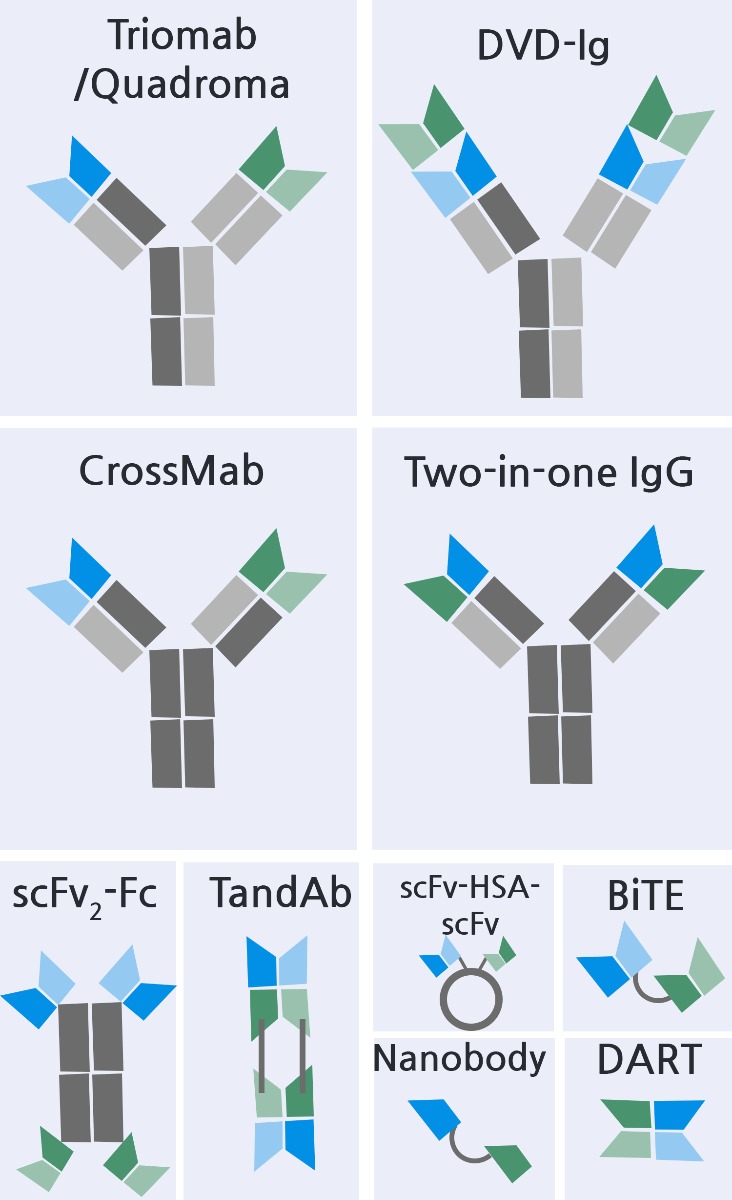
An antibody (Ab) containing two different antigen-binding sites within one molecule is known as a bispecific antibody (BsAb). BsAb research has expanded greatly within the past ten years. To date, two BsAbs, Blinatumomab and Emicizumab, have been approved in the US and the EU, while a third BsAb, Catumaxomab, is already commercially available in the EU. Additionally, there are currently more than 60 BsAb drugs in preclinical trials, and another 30 entered in clinical trials. Of these, two thirds focus on the treatment of cancer by means of bringing effector T lymphocytes (or natural killers) closer to target cells that express specific cancer antigens on their surface.
Blinatumomab - Mechanism of Action
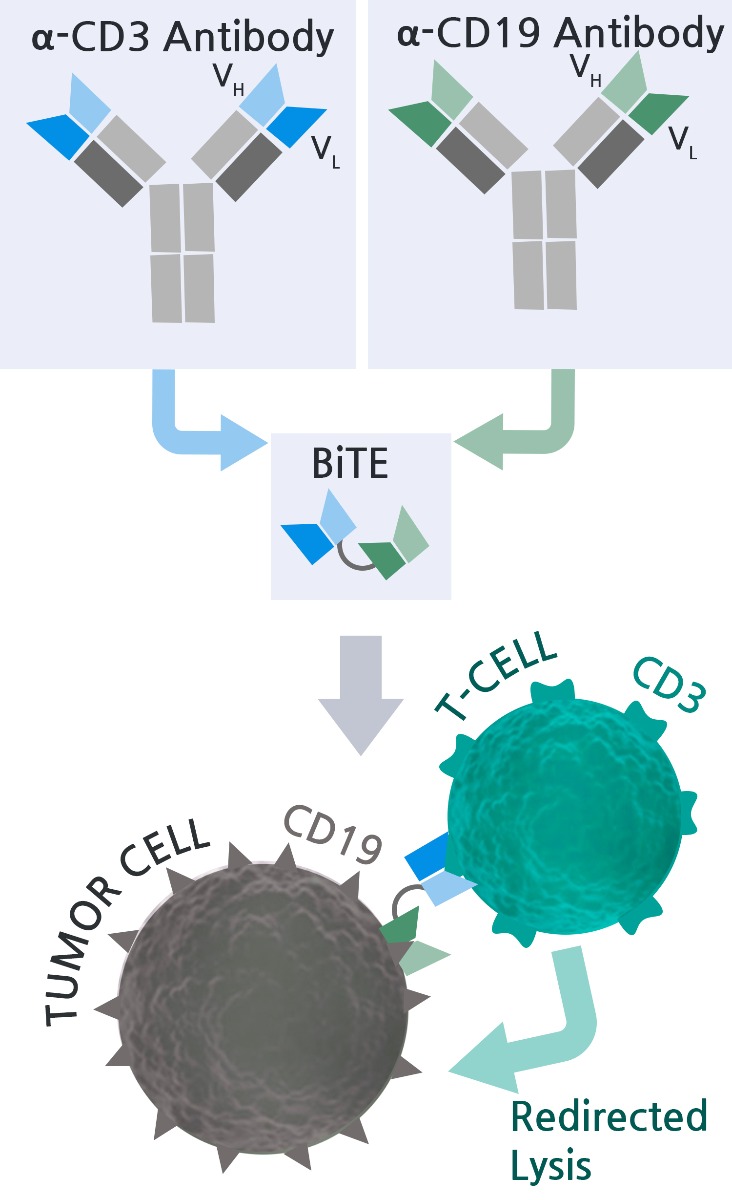
Blinatumomab is a biopharmaceutical drug used as a second-line treatment for non-Hodgkin’s lymphoma or refractory acute lymphoblastic leukemia. It is a Bispecific T-Cell Engager (BiTE®), a small fusion protein containing two antibody binding sites. One site binds to CD19 on cancer cells (e.g. Raji cells) and the other binds simultaneously to CD3 on T cells, thus linking the T lymphocytes to cancer cells expressing specific cancer antigens (e.g. CD19). The binding event potentiates unstimulated T cells and induces direct cytotoxicity against CD19+ cancer cells.
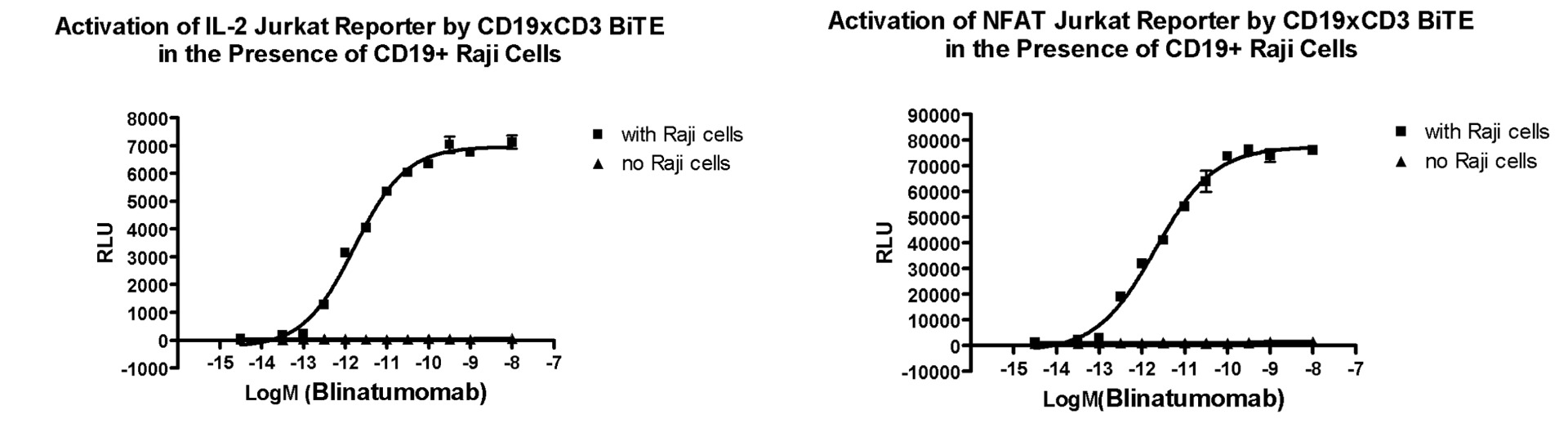
BPS has produced a CD19/CD3 BiTE® that is functionally identical to Blinatumomab. The ability of CD19/CD3 BiTE® to bind and activate T cells has been validated using several reporter cell lines. The CD19/CD3 BiTE® is a useful tool for studying CD19+ cancer cell-mediated T cell activation, using either primary T cells or reporter cell lines such as Jurkat/NFAT-luc or Jurkat/IL2-Luc.
In relation, BPS has developed an in-vitro binding assay kit for Blinatumomab and biotinylated CD19 to show a Kd value in the low nM range.
Experimental Procedures
Jurkat effector cells with endogenous TCR/CD3 and stably transfected reporter NFAT-Luciferase or IL-2 promoter-Luciferase are incubated with increasing concentrations of CD19/CD3 BiTE®in the presence or absence of CD19+ Raji cells. The CD19/CD3 BiTE® simultaneously binds to TCR/CD3 on the Jurkat reporter cells and tumor antigen CD19 on the target Raji cells. This binding stimulates NFAT- or IL-2-induced luciferase expression and the luciferase activity is assessed by our ONE-Step™ Luciferase Assay System.
BsAb-related BPS Products and Services
BPS Bioscience offers customized services for the production of high-quality bispecific antibodies and BiTE®, determination of Kd values using in vitro assay kits, and assessment of T cell activation using cell-based assays. BPS also offers over 200 recombinant cell lines expressing antigens on cancer cells including our CD19-CHO Cell Line and high titer lentiviruses of reporter genes including the NFAT Luciferase Reporter Lentivirus.
If you have further questions or would like to request a quote, please feel free to contact us.
References
1. Labrijn, A.F., Janmaat, M.L., Reichert, J.M., and Parren, P.W.H. (2019) Bispecific antibodies: a mechanistic review of the pipeline, Nature Reviews Drug Discovery 19, 585-608
2. Sedykh, S.E., Prinz, V.V., Buneva, V.N., and Nevinsly, G.A. (2018) Bispecific antibodies: design, therapy, perspectives, Drug Design, Development and Therapy 12, 195-208