SARM1, FLAG-Tag Recombinant
Catalog #
100069
$585
*
●
●
Purchase
Description
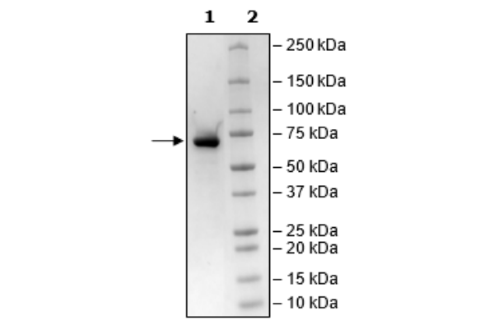
Recombinant human SARM1 (sterile alpha and TIR motif containing 1), encompassing amino acids 28-724 (end). This construct contains an N-terminal FLAG tag. The protein was affinity purified.
●
Synonyms
Human SARM1, also known as Sterile Alpha And TIR Motif Containing 1, and SAMD2, NAD(+) hydrolase SARM1, HsTIR, hSARM1, MyD88-5, NADase SARM1, SARM-1
●
Product Data Gallery
Product Info
Storage and Usage
Citations
Species
Human
Construct
SARM1 (FLAG-28-724(end))
Host Species/Expression System
HEK293
Purity
≥90%
Format
Aqueous buffer solution.
Formulation
40 mM Tris-HCI, pH 8.0, 110 mM NaCl, 2.2 mM KCl, 20% glycerol, 3 mM DTT, 100 µg/ml FLAG peptide.
MW
77 kDa
Amino Acids
28-724(end)
Genbank #
NM_015077
UniProt #
Q6SZW1