IL-23A:IL-23R Assay Service
●
Target
IL-23A:IL-23R
●
Description
Screening and/or profiling compounds or biologics that inhibit IL-23A binding to IL-23R in a biochemical assay.
●
Synonyms
Interleukin 23 subunit alpha, IL-23p19, IL-23R
●
Example Data
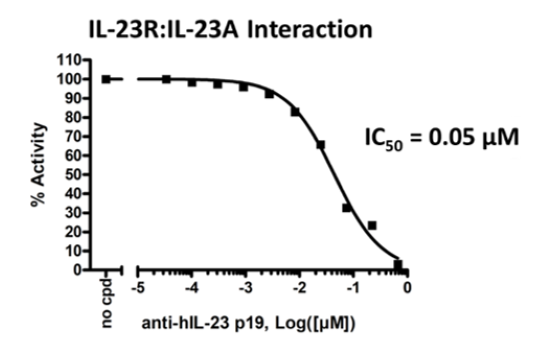
*Example only, final data may vary.
Assay Details
●
Assay Format
Chemiluminescent
●
Reference Compounds and IC50
Anti-IL-23A Antibody, 50 nM
●
Assay Principle
The IL-23RA:IL-23A[Biotin] Inhibitor Screening Assay is designed for screening inhibitors of IL-23A:IL-23RA interaction. The key to this kit is the high sensitivity of detection of biotin-labeled IL-23A by streptavidin-HRP. Only a few simple steps on a microtiter plate are required for the assay. First, IL-23RA is coated on a 96-well plate. Next, IL-23A[Biotin] is incubated with IL-23RA on the plate. Finally, the plate is treated with streptavidin-HRP followed by addition of an HRP substrate to produce chemiluminescence, which is then measured using a chemiluminescence reader.
Target Details
●
Protein Family
Immunotherapy
●
UniProt
Q9NPF7, Q5VWK5
●
Background
IL-23 (interleukin 23) is a cytokine involved in inflammation. It is composed of two subunits, IL-12B (or IL-12p40) and IL-23A (or IL-23 p19) and binds to the IL-23 receptor. It supports Th17 T cell maintenance, expansion and cytokine release, NK cell IFNγ (interferon γ) secretion and increased ADCC (antibody-dependent cellular cytotoxicity) and CD4+ T cell proliferation. IL-23 is produced and secreted by activated dendritic cells, macrophages, B cells and γδ T cells. Excessive production of this cytokine can lead to autoimmune disorders, such as psoriasis, and even cancer. Ustekinumab (sold under the brand name STELARA®) and guselkumab (sold under the brand name Tremfya®) are two commercial monoclonal antibodies targeting IL-23, designed for the treatment of Crohn’s disease, ulcerative colitis, plaque and arthritic psoriasis. The success of these two drugs indicates the relevance of this cytokine in human health and disease, making it a valuable therapeutic target.
Delivery
●
Estimated Turnaround
Two to three weeks following delivery of compounds
●
Results
Extensive report with raw and analyzed data, graphs, and detailed protocols. Includes positive control for inhibition.

