GLP-1R and Diabetes
GLP-1R Structure and Function
Glucagon like peptide-1 receptor (GLP-1R) is a member of the Class B GPCR family (1). Human GLP-1R comprises 463 amino acids, including seven transmembrane domains and an N-terminal signal peptide that is cleaved upon delivery to the plasma membrane (2). The hallmark structural feature of GLP-1R and other Class B GPCRs is a large N-terminal extracellular domain (ECD). Class B GPCRs typically bind their peptide ligands through a mechanism known as the two-domain model, in which ECD binds to the C-terminal helix of GLP-1, and the transmembrane domain (TMD) binds to the N-terminal region of GLP-1 (3, 4, 5). It is known that the polar residues that reside within the TMD regulate biased signaling of the receptor (3), while the transmembrane helical boundaries and extracellular surface are a trigger for biased agonism (4). GLP-1R is abundantly expressed in the pancreas and CNS. It is also expressed at lower levels in the gut, kidneys, liver, heart, muscle and the PNS (6).
Glucagon like peptide-1 (GLP-1) is the natural ligand of GLP-1R. It is a 31 amino acid long peptide hormone (7) mainly secreted by enteroendocrine L cells in the distal intestine, alpha cells in the pancreas, and by the preproglucagon (PPG) neurons located in the nucleus of the solitary tract of the caudal brainstem (6). GLP-1 interacts with its receptor GLP-1R and regulates glucose homeostasis. Therefore, it’s a highly attractive target for type 2 diabetes (T2D) therapy. GLP-1 or GLP-1R agonists display a diverse range of physiological functions as shown in the image below.
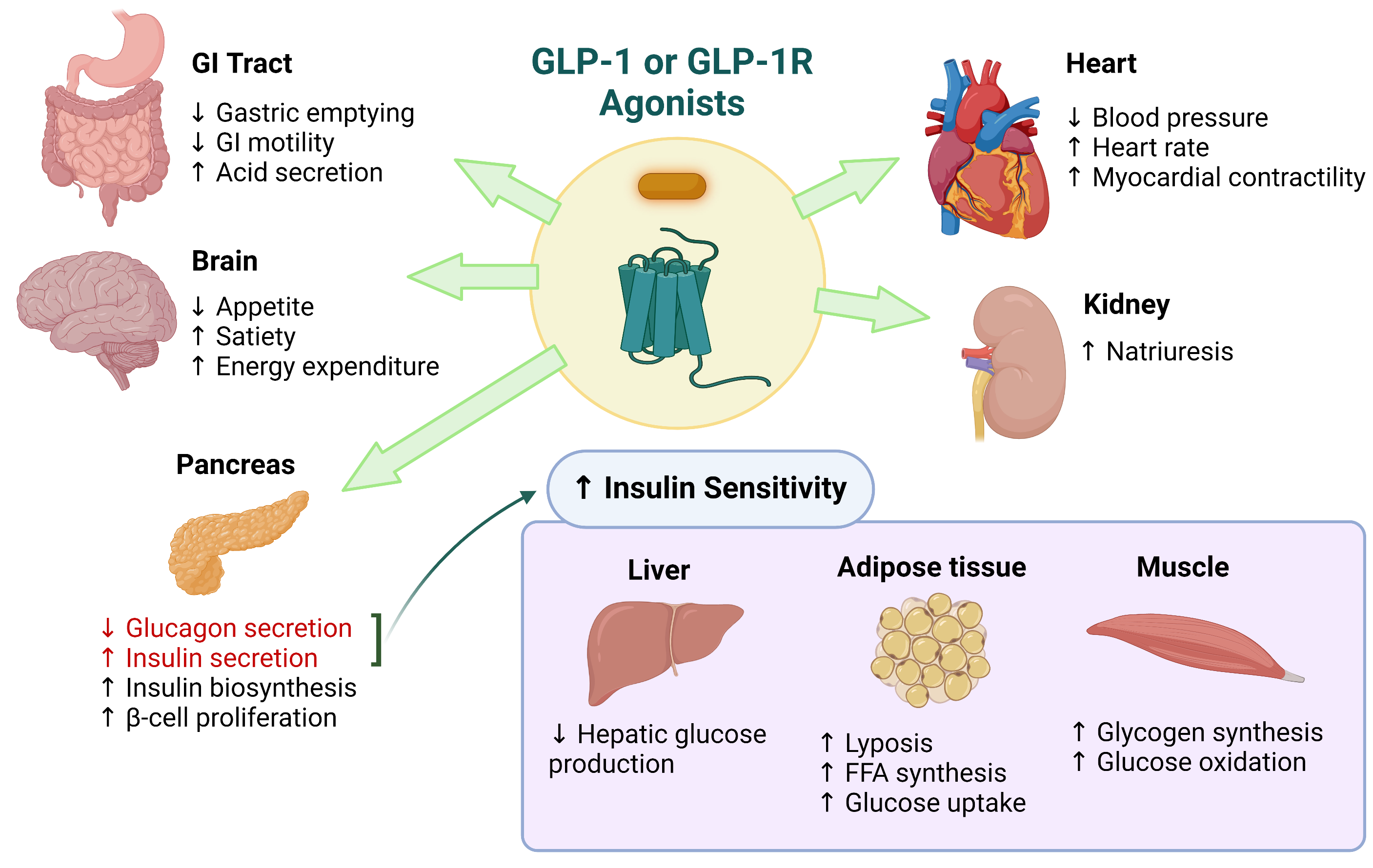
Image adapted from: Saraiva, FK and Sposito, A. Cardiovascular Diabetology. 2014, 13:142 http://www.cardiab.com/content/13/1/142.
GLP-1R Signaling
Binding of GLP-1 to GLP-1R triggers a downstream signaling cascade that induces a potent stimulation of glucose induced insulin secretion (GIIS) in pancreatic beta-cells, as well as inhibition of alpha cell glucagon release (6). Downstream activation of GLP-1R results in rapid activation of adenyl cyclase and increased cAMP secretion. cAMP directly activates protein kinase A (PKA) and cAMP-regulated guanine nucleotide exchange factor 2 (Epac2), that act synergistically to generate downstream signals resulting in increased insulin secretion (8). Activation of GLP-1R also increases intracellular Ca2+ through Ca2+ release from the endoplasmic reticulum (ER) via the inositol 1,4,5 triphosphate receptors activated by PKA and the ryanodine receptors activated by Epac2 (9). Hence, GLP-1R is able to couple not only to Gαs, but also to other Gα subtypes including Gαi and Gαq. GLP-1R also signals via the G-protein independent (β-arrestin-mediated) pathway.
GLP-1 signaling also antagonizes voltage-dependent K+ (Kv) channels via cAMP/PKA-dependent pathway in beta cells, which prevents beta-cell repolarization by reducing Kv currents. This enables voltage-dependent Ca2+ channels (VDCCs) to open and the influx of Ca2+ results in the exocytosis of insulin from β-cells (13). Therefore, the combination of both cAMP production and influx of Ca2+ are vital components in the biosynthesis and secretion of insulin (10).
GLP-1R as a Target for Diabetes and Other Diseases
Type 2 diabetes also known as adult-onset diabetes is the most common form of diabetes in human populations, estimated to affect over 450 million individuals, corresponding to over 6% of the world’s population. It arises due to a combination of genetic and lifestyle factors that contribute to defective production of insulin from pancreatic beta cells, and/or resistance to its uptake by insulin receptors located in peripheral and central tissues in the body. Prolonged elevation of blood glucose, as well as related adverse metabolic features such as dyslipidemia and chronic inflammation, lead ultimately to a range of debilitating health issues such as, cardiovascular disease, peripheral neuropathy, diabetic ketoacidosis, diabetic retinopathy, and diabetic nephropathy. Existing anti-diabetic drugs contribute towards improving glycemic control by either increasing the body’s sensitivity to insulin, enhancing insulin secretion or reducing renal glucose reabsorption. However, these drugs are known to cause a plethora of adverse effects such as weight gain, edema, intestinal discomfort and hypoglycemia (2).
Recently, glucagon-like peptide 1 receptor agonists (GLP- 1RAs) have emerged as safe and effective treatments for diabetes and obesity. GLP-1R, when activated, initiates downstream signaling effects that lead to an overall decrease in blood glucose levels, including potentiation of insulin secretion, reduced glucagon secretion, and weight loss via satiety induction that results in increased insulin sensitivity (2). GLP-1RAs work to stimulate insulin secretion in a glucose-dependent manner, thus carrying a low risk of hypoglycemia. To date, they represent the only G protein-coupled receptor (GPCR) ligands approved as glucose-lowering agents (2).
A variety of GLP-1RAs and analogs, such as Exendin-4 and Liraglutide, are currently being used to successfully treat T2D (7). In 2014, Liraglutide became the first GLP-1RA medication to be introduced to the US market for treatment of obesity and weight management (12). The therapeutic benefits of GLP-1RA go beyond T2D and obesity treatment. GLP-1RAs have shown to improve cardiovascular health by decreasing blood pressure, stimulate differentiation of nerve cells and inhibit neuroinflammation, and inhibit hepatitis (7). GLP-1 and GLP-1RAs have also shown to exert anti-depressive effects by reversing many maladaptive brain changes relevant to depression (13).
In addition to the US FDA approved GLP-1RAs, there are GLP1/glucagon dual agonists (Cotadutide, BI 456906), GIP/GLP1 dual agonists (Tirzepatide, GIP/GLP peptide I and II), GIP/GLP1/glucagon tri-agonists (HM15211, GGG tri-agonist) and GLP1R agonists (Efpeglenatide, Rybelsus) currently in phase 1- III clinical trials for T2D, obesity, and NASH (MASH) treatments (12). GIP (Glucose-dependent insulinotropic polypeptide or gastric inhibitory polypeptide) is an intestinal hormone that acts on its receptor, GIP-R, to stimulate insulin secretion. Therefore, GLP-1RAs and GIP-RAs hold promise to be potential treatments for a wide variety of diseases.
Featured product:
GLP-1R/CRE (Luc) Reporter – HEK293 Recombinant Cell Line
This HEK293 cell line is engineered to produce a robust luciferase readout upon stimulation of the GLP-1R at the cell surface. It is tested using recombinant GLP-1, as well as several GLP-1R agonists. The cell line is ideal for screening candidate molecules for GLP-1 agonist activity and determining EC50 dosing.
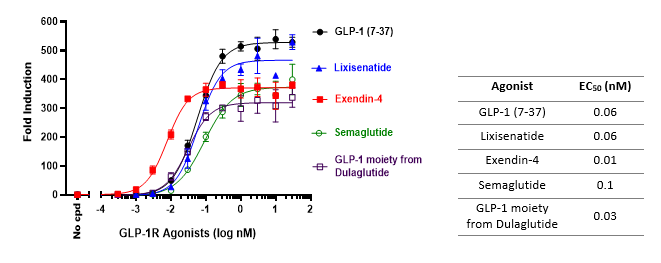
GLP-1R and GIPR Agonist Screening & Profiling Services
Do you need help with testing and screening your agonist compounds with our cell lines? We provide convenient, fast turnaround, and reliable results with our expert screening team. Inquire with us today!
References:
(1) Dillon JS, et al. 1993. Endocrinology. 133 (4):1907–10.
(2) Marzook A, et al. 2021. Front Endocrinol. 12:678055. doi: 10.3389/fendo.2021.678055
(3) Wootten D, et al. 2016. Molecular Pharmacology. 89 (3):335–47.
(4) Wootten D, et al. 2016. Cell. 165 (7):1632–1643. doi:10.1016/j.cell.2016.05.023
(5) Yang D, et al. 2016. J Biol Chemistry. 291 (25):12991–3004.
(6) Rowlands J, et al. 2018. Front Endocrinol. 9:672. doi: 10.3389/fendo.2018.00672
(7) Zhao X, et al. 2021. Front Endocrinol. 12:721135. doi: 10.3389/fendo.2021.721135
(8) Carlessi R, et al. 2017. Sci Rep 7, 2661.
(9) Portha B, et al. 2011. Exp Diabetes Res. 2011:376509. doi: 10.1155/2011/376509.
(10) Cassandra Koole, et al. 2013. Molecular Endocrinology, 27:1234–1244.
(11) https://www.who.int/news-room/fact-sheets/detail/diabetes
(12) Müller TD, et al. 2022. Nat Rev Drug Discov. 21:201–223 (2022).
(13) Detka J, and Głombik K. 2021. Pharmacol Rep. 73:1020–1032.

