NF-κB Luciferase Reporter Jurkat Cell Line
The NF-κB Luciferase Reporter Jurkat Cell Line is a Jurkat cell line designed for monitoring nuclear factor Kappa B (NF-κB) signal transduction pathways. It contains a firefly luciferase reporter driven by four copies of the NF-κB response element located upstream of the minimal TATA promoter. After activation by pro-inflammatory cytokines or activators of lymphokine receptors, endogenous NF-κB transcription factor binds to the DNA response elements, inducing transcription of the luciferase reporter.
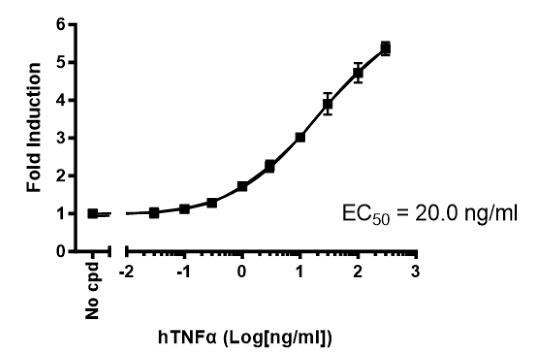
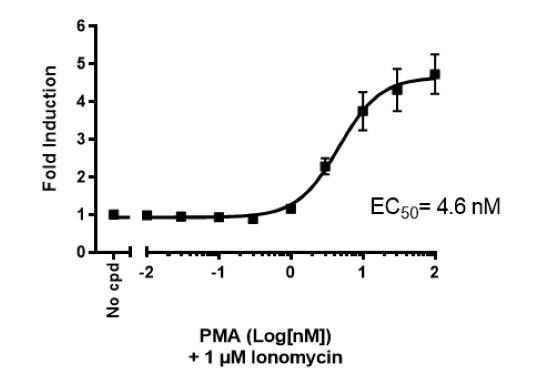
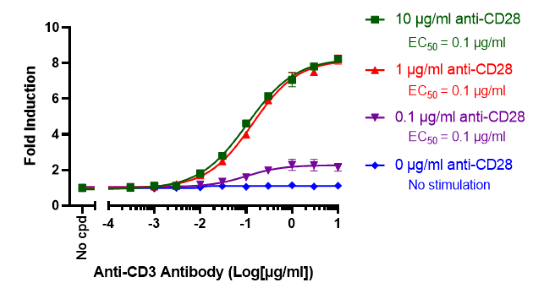
This cell line has been functionally validated and responds to phorbol 12-myristate 13-acetate (PMA) with ionomycin and TNFα. It can be used to measure TCR (T Cell Receptor)-mediated T cell activation through co-stimulation with anti-CD3 and anti-CD28 antibodies
Interested in screening and profiling inhibitors, blocking antibodies, or activators of NF-κB-mediated signaling without the need to purchase and license the cell line? Check out our Cell Signaling Pathway Screening.
This product has been cited 9 times.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
Media Required for Cell Culture
| Name | Ordering Information |
| Thaw Medium 2 | BPS Bioscience #60184 |
| Growth Medium 2B | BPS Bioscience #79530 |
Materials Required for Cellular Assays
| Name | Ordering Information |
| Ionomycin | Sigma #I3909 |
| Phorbol 12-myristate 13-acetate (PMA) | LC Laboratories #P1680 |
| TNFα | Sigma #T0157-10UG |
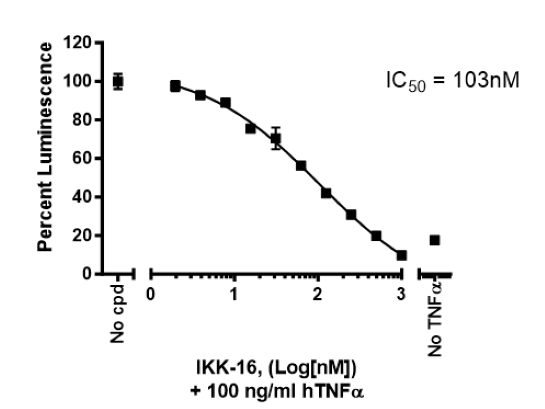
| IKK-16 dihydrochloride: inhibitor of NF-κB activation | Sigma #SML1138 |
| Anti-CD3 Agonist Antibody | BPS Bioscience #71274 |
| Anti-CD28 Agonist Antibody | BPS Bioscience #100182 |
| ONE-Step™ Luciferase Assay System | BPS Bioscience #60690 |
| White, clear-bottom cell culture plate, 96-well | |
| Luminometer |
The cell line has been screened to confirm the absence of Mycoplasma species.
Nuclear factor-Kappa B (NF-κB)/Rel proteins include NF-κB2 p52/p100, NF-κB1 p50/p105, c-Rel, RelA/p65, and RelB. These proteins function as dimeric transcription factors that control genes regulating a broad range of biological processes including innate and adaptive immunity, inflammation, stress responses, B cell development, and lymphoid organogenesis. In the classical (or canonical) pathway, NF-κB/Rel proteins are bound and inhibited by IκB proteins. Proinflammatory cytokines, growth factors, and antigen receptors activate an IKK (inhibitor of κB kinase) complex (IKKβ, IKKα, and NEMO (NF-κB essential modulator)), which phosphorylates IκB proteins. Phosphorylation of IκB leads to its ubiquitination and proteasomal degradation, freeing NF-κB/Rel complexes. Free NF-κB/Rel complexes are further activated by phosphorylation and translocated to the nucleus where they induce the expression of target genes. In the alternative (noncanonical) NF-κB pathway, NF-κB2 p100/RelB complexes are inactive in the cytoplasm. Signaling through a subset of receptors including LTβR (lymphotoxin beta receptor), CD40, and BR3 (Blys receptor 3), activates the kinase NIK (NF-κB-inducing kinase), which in turn activates IKKα complexes that phosphorylate C-terminal residues in NF-κB2 p100. Phosphorylation of NF-κB2 p100 leads to its ubiquitination and proteasomal processing to NF-κB2 p52, creating transcriptionally competent NF-κB p52/RelB complexes that translocate to the nucleus and induce target gene expression. An understanding of the NF-κB pathway and how to modulate is critical to understand gene regulation in health and disease.
Clipstone N.A. and Crabtree G.R., 1992 Nature. 357(6380):695-7.
Lyakh, L., et al., 1997 Mol Cell Biol. 17(5):2475-84.