IgG Antibodies and Fcγ Receptors in Immunotherapy
IgG Antibodies and Fcγ Receptors in Immunotherapy
Monoclonal, immunoglobulin G (IgG), antibodies are the basis of powerful therapies for treating cancer, viral infections, and autoimmune disease. The power of therapeutic antibodies comes from their ability to bind antigen targets with high specificity and then harness the functions of the larger the immune system. There are a variety of immune responses. Cytotoxic antibodies directly deplete cancer cells. Antibodies that target immune checkpoint inhibitors modulate cellular populations in the tumor microenvironment. Anti-inflammatory antibodies directly target cytokine and cytokine receptors to treat autoimmune disease. IgG antibodies regulate this variety through a common mechanism of control.
IgG antibodies regulate the immune system by binding specific receptors (Fcγ receptors, FcγRs), on the surface of immune cells. The interactions with these receptors occurs through fragment crystallizable (Fc) region of the antibody. The molecular composition and structure of the Fc region determines which receptors are bound leading to specific immune responses.
The design Fc regions to engage the proper FcγR for optimal response is a major component in the development of next generation therapies. This requires an understanding the molecular structure and function of both antibody and receptor. It also requires the proper tools and specialized reagents for efficiently conducting optimization experiments.
Molecular Structure of IgG Antibodies
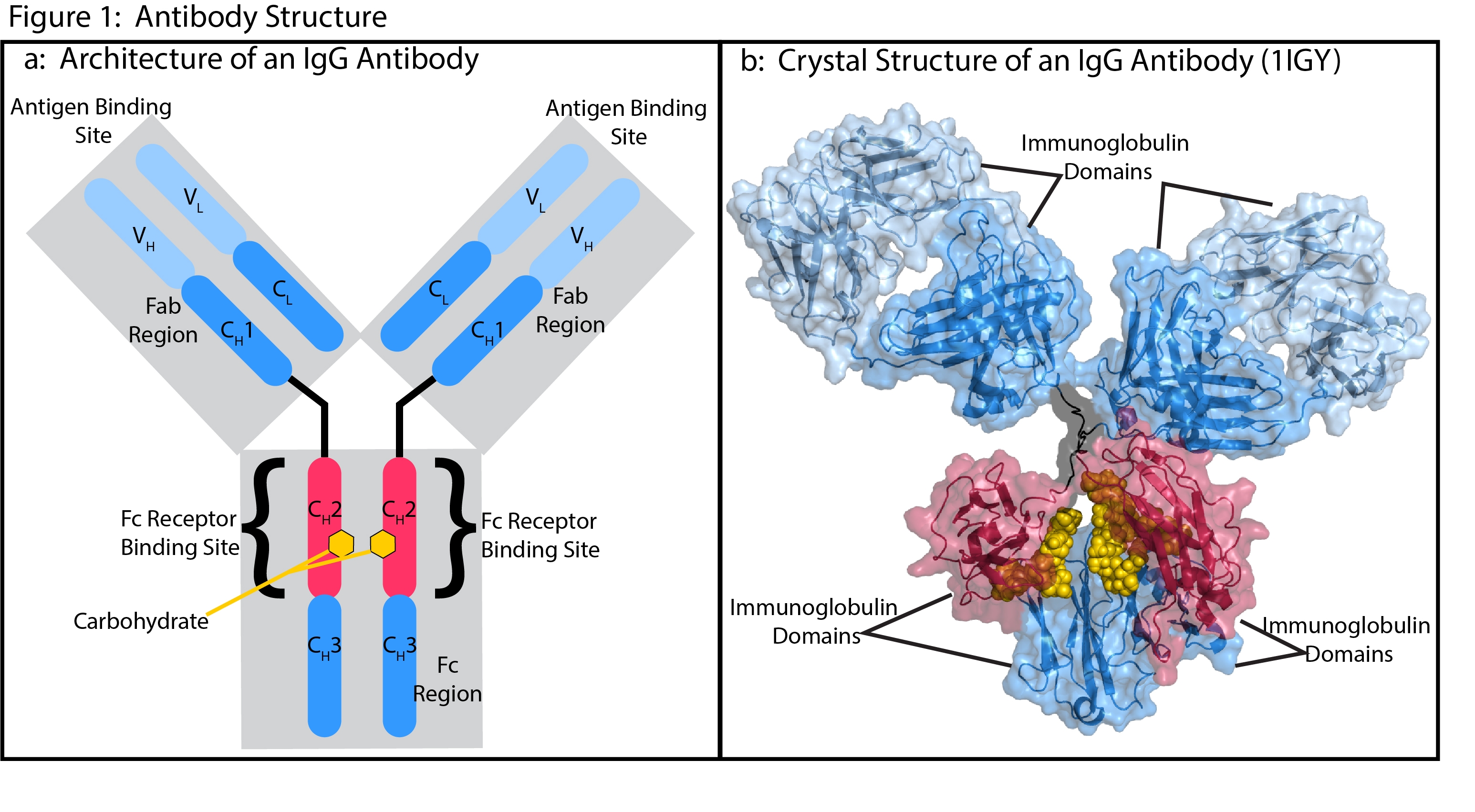
IgG antibodies have one of the most thoroughly characterized structures in biology1, 2 (Fig 1). The general structure consists of multiple regions defined by classical mapping studies of susceptibility to cleavage by proteases. The main regions include two fragment antigen binding (Fab) regions that make up the arms of the classic Y shape and the Fc region, which forms the stem.
Antibodies are comprised of four protein chains that include two identical light chains and two identical heavy chains. These chains are connected through noncovalent interactions and crosslinked by pairs of conserved disulfide bonds. Each chain consists of conserved (CH1-3 and CL) and variable (VH and VL) sequences that fold into a similar structure known as the immunoglobulin domain. The combination of variable and conserved sequences in a Fab make up the antigen binding site with the variability of the V regions allowing the specificity of antigen binding.
The Fc receptor binding site and the carbohydrate attachment sites are in the CH2 segment of the Fc region. The four classes of IgG subtypes differ in their CH2 sequences and carbohydrate attachment sites, which enables different effector functions for each IgG subtype3. Much of the work in developing therapeutic antibodies involves optimizing the sequences of the Fc domain and the attached carbohydrates to produces desirable effector functions through interactions with specific FcγgRs.
Structure and Function Of Type I FCγRs
FcγRs fit into two major classes (type I and type II), based on their extracellular structure, their binding stoichiometry with the Fc domain, and their intracellular signaling domains1. The majority of therapeutic antibodies interact with type I receptors. This makes them the particular focus in immunotherapy drug discovery and development.
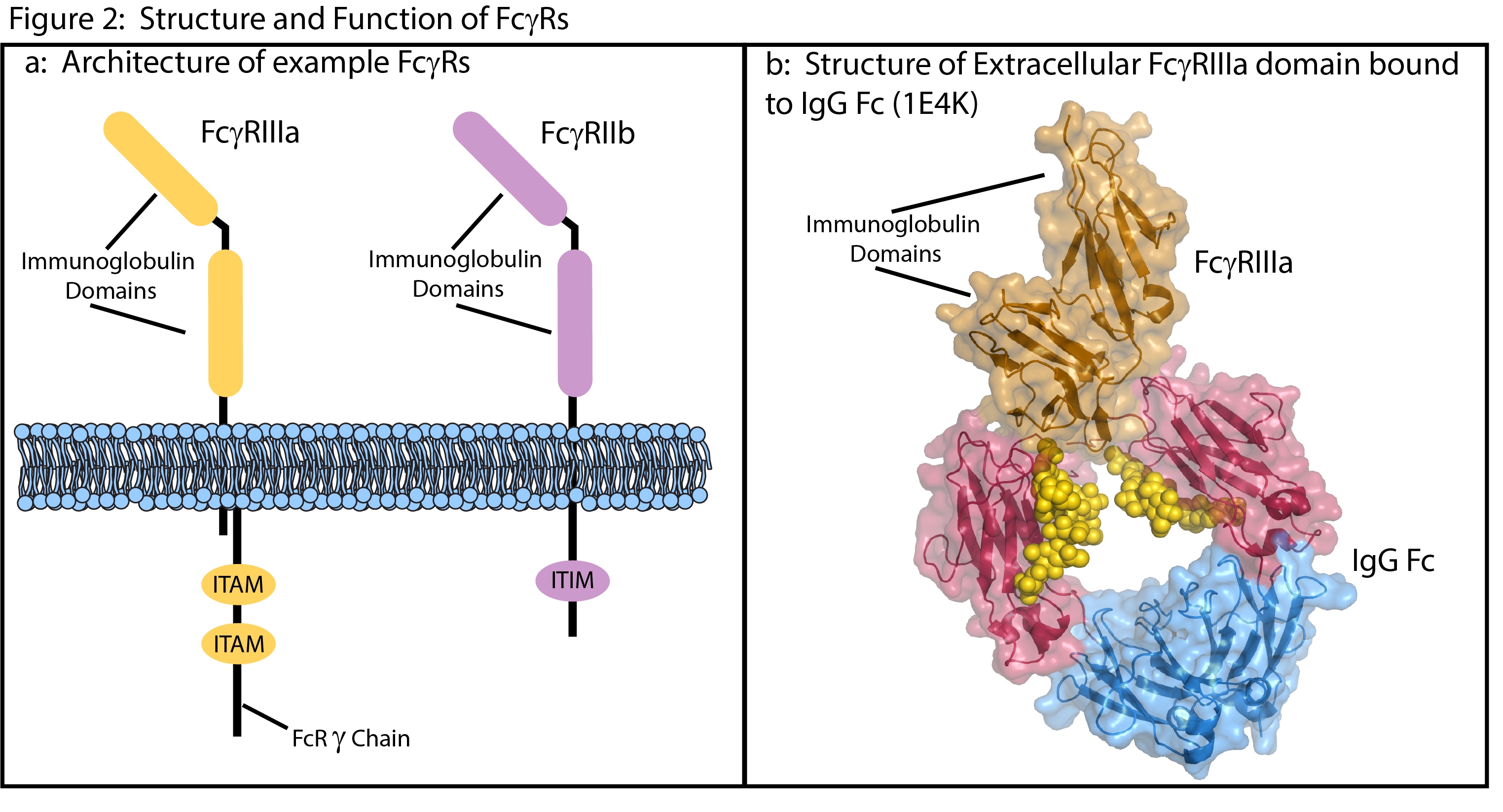
The subtypes of Type I receptors have broad similarities in structure and cell signaling mechanisms. Their extracellular domains contain multiple immunoglobulin domains that bind the Fc region of antibodies with a 1 to 1 stoichiometry1, 4 (Fig 2). These are low affinity (µM to high nM) interactions driven by high-avidity clustering on multimeric immune-complexes5.
The majority of Type I receptors are immune activators that signal through either intracellular, immunoreceptor tyrosine-based activation motifs (ITAM) or through ITAMs on noncovalently attached FcRg[S1] chains6. However, FcγRIIb acts as an immune-inhibitor Type I receptor, signaling through an intracellular immunoreceptor tyrosine-based inhibitor motif (ITIM)6.
Most types of immune effector-cells express both stimulatory and inhibitory Type I receptors6. The distinct functions of these molecules are largely regulated by the selective binding of IgG subtypes. The broad expression patterns of Type I receptors and the variety of IgG subtypes can lead to completely opposing signals, making it essential to consider FcγgR interactions in antibody design.
Fc Gamma Receptor IIIA (FcγRIIIA) in Antibody-dependent cell-mediated cytotoxicity (ADCC)
Antibody-dependent cell-mediated cytotoxicity (ADCC) is a powerful effector function where natural killer (NK) cells lyse a target cell that is covered with IgG antibodies bound to antigens in its cell membrane7. NK cells express the type I receptor FcγRIIIa (CD16a), and Fc stimulation of this receptor signals the releases cytokines and cytotoxic molecules that then attack the target cell7.
The human FcγRIIIa is dimorphic at residue 158 and this variation may be important for the development of new treatments8-11. The V158 variant has a higher affinity for IgG1 and IgG3 than the other common variant, F158. A higher response rate to therapeutic antibodies for breast cancer, lymphoma, and colorectal cancer is observed in patients carrying V1588-11. Intense research is focused on development of antibodies with improved Fc binding to one or both receptor variants to provide a more personalized approach to treatment.
BPS Bioscience has created cell-based reporter systems to aid in the optimization of ADCC-based therapeutic antibodies. These include recombinant Jurkat T cells expressing firefly luciferase gene under the control of NFAT response elements, along with constitutive expression of either human FcγRIIIa, low affinity (F158) (#60540) variant or high affinity variant (V158) (#60541). When combined with our ONE-Step™ luciferase assay system, these cell lines provide a reliable tool for assessing FcγRIIIa interactions with antibody-coated cells.
Fc Gamma Receptor 2B (FcγRIIB) Targeting: an Immune Inhibitor
FcγRIIB is the only type I receptor that is a negative regulator of the immune system1, 12. In healthy people it is expressed on B cells and functions in the regulation of antibody production. However, interaction with FcγRIIB negatively impacts treatment with therapeutic antibodies. Outcomes may be improved by preventing immune inhibition caused by Fc binding to FcyRIIB.
FcγRIIB is also a direct therapeutic target12. Antibodies targeting FcγRIIB directly can promote immune attack or they may induce apoptosis on B cell-derived cancer cells. Cross-linking FcγRIIB is also being researched as a treatment for allergic reactions and other autoimmune diseases12.
Our FcγRIIB cell line (#79511) stably expresses full length human FcγRIIB. Crosslinking of antibodies to FcγR can promote receptor clustering and increase downstream signaling in antigen-expressing reporter cell lines. Combining these cells with reporter cells expressing CD137 (#79289) or other checkpoint inhibitors provides a powerful tool for quantifying antibody interactions with FcγRIIB.
BPS Bioscience Is Your Partner In Fcγ Receptor and IgG Therapeutic Antibody projects
Antibody interactions with FcγRs are a critical and fascinating part of the development of new therapeutics. BPS Bioscience can provide the tools and project guidance for both custom projects and fully developed research products. Contact us today to learn more.
References
[1] Bournazos, S., and Ravetch, J. V. (2017) Fcgamma Receptor Function and the Design of Vaccination Strategies, Immunity 47, 224-233.
[2] Harris, L. J., Skaletsky, E., and McPherson, A. (1998) Crystallographic structure of an intact IgG1 monoclonal antibody, J Mol Biol 275, 861-872.
[3] Wang, Q., Chung, C. Y., Chough, S., and Betenbaugh, M. J. (2018) Antibody glycoengineering strategies in mammalian cells, Biotechnol Bioeng 115, 1378-1393.
[4] Sondermann, P., Huber, R., Oosthuizen, V., and Jacob, U. (2000) The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex, Nature 406, 267-273.
[5] Duchemin, A. M., Ernst, L. K., and Anderson, C. L. (1994) Clustering of the high affinity Fc receptor for immunoglobulin G (Fc gamma RI) results in phosphorylation of its associated gamma-chain, J Biol Chem 269, 12111-12117.
[6] Nimmerjahn, F., and Ravetch, J. V. (2005) Divergent immunoglobulin g subclass activity through selective Fc receptor binding, Science 310, 1510-1512.
[7] Ochoa, M. C., Minute, L., Rodriguez, I., Garasa, S., Perez-Ruiz, E., Inoges, S., Melero, I., and Berraondo, P. (2017) Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells, Immunol Cell Biol 95, 347-355.
[8] Bibeau, F., Lopez-Crapez, E., Di Fiore, F., Thezenas, S., Ychou, M., Blanchard, F., Lamy, A., Penault-Llorca, F., Frebourg, T., Michel, P., Sabourin, J. C., and Boissiere-Michot, F. (2009) Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan, J Clin Oncol 27, 1122-1129.
[9] Cartron, G., Dacheux, L., Salles, G., Solal-Celigny, P., Bardos, P., Colombat, P., and Watier, H. (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene, Blood 99, 754-758.
[10] Musolino, A., Naldi, N., Bortesi, B., Pezzuolo, D., Capelletti, M., Missale, G., Laccabue, D., Zerbini, A., Camisa, R., Bisagni, G., Neri, T. M., and Ardizzoni, A. (2008) Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer, J Clin Oncol 26, 1789-1796.
[11] Wen, Y. M., Mu, L., and Shi, Y. (2016) Immunoregulatory functions of immune complexes in vaccine and therapy, EMBO Mol Med 8, 1120-1133.
[12] Smith, K. G., and Clatworthy, M. R. (2010) FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications, Nat Rev Immunol 10, 328-343.
Whatever your research project may entail, BPS Bioscience is here to help you meet your goals. Contact us today to learn more.