Sortase-Mediated Protein Ligation
Protein modification is a powerful tool for both research and medicine, and these modifications are of special importance to the research and drug discovery communities. Proteins modified with added fluorophores or other compounds are instrumental in studying cellular pathways and molecular mechanisms 1-4. Additionally, antibodies conjugated with toxic cargos can be highly potent anti-tumor drugs5-7. At BPS, we specialize in numerous methods of protein modification that are useful for both assay development and custom services.
Modification is often accomplished through chemical conjugation of lysine or cysteine side-chains. Challenges often arise from non-specific modifications that can block important elements of the protein. Nonspecificity also impacts reproducibility by generating proteins that are heterogeneous mixtures of labeling sites and degrees of conjugation. To minimize these issues, we developed methods of site-specific conjugation using the Sortase A enzyme that provide efficient modification of target proteins without the complications associated with nonspecific conjugation.
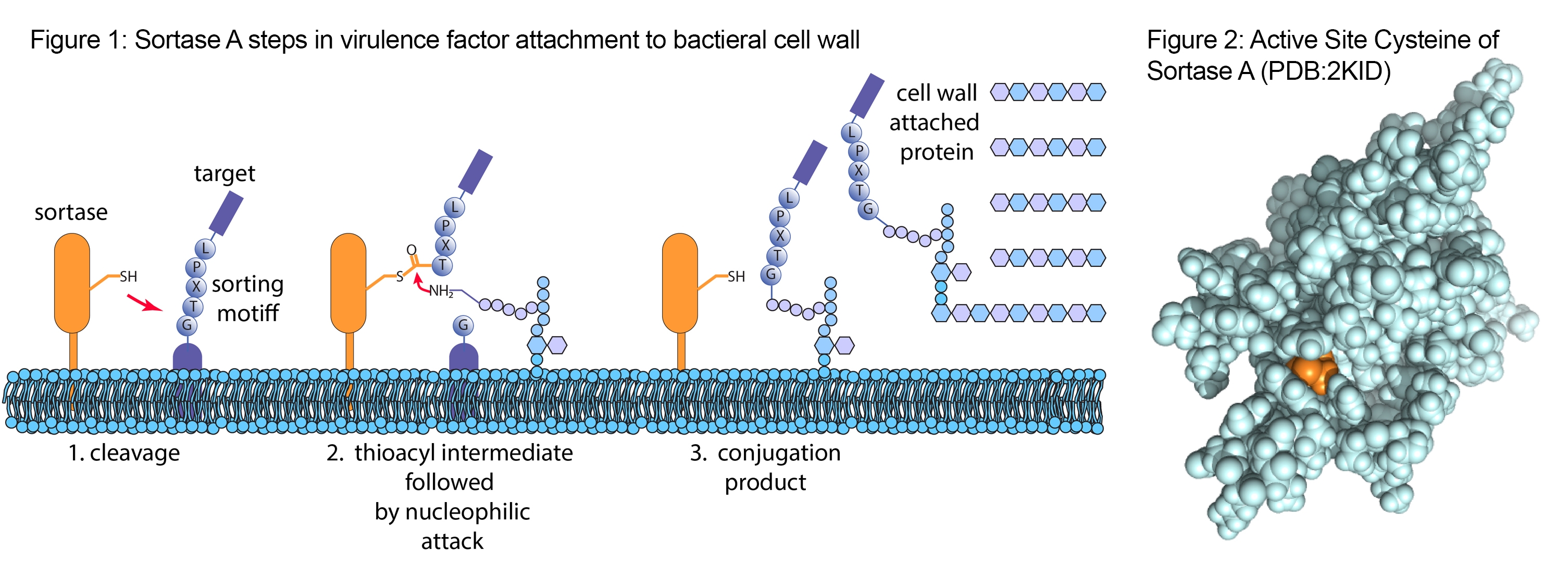
Sortases are transpeptidases essential for cell wall biosynthesis and bacterial virulence of Gram-positive bacteria 8-11. Sortase A from Staphylococcus aureus is well-characterized and is a useful tool for generating protein modifications. In nature, it attaches virulence factors to the bacterial cell wall by recognizing a core LPXTG motif and cleaving the amide bond between the threonine and glycine residue (Fig 1). The active site cysteine (orange in Fig 2) [S1] [B2] generates a semi-stable thioacyl intermediate, covalently linking Sortase A to its substrate. This intermediate then undergoes nucleophilic attack by the α-amine of the oligoglycine group present on lipid II, resulting in the new peptide bond formation between the substrate protein and lipid II.
The transpeptidase activity of Sortase A is especially useful for labeling because it will work on almost any protein bearing the LPXTG recognition motif. Once the sorting motif is included in the protein of interest, all one needs is Sortase A and an oligoglycine nucleophile containing a payload of choice. This system is perfect for a number of diverse applications, including labeling proteins with small fluorescent probes, generating non-natural protein dimers and cyclic proteins, and immobilizing proteins to solid surfaces12. Additionally, Sortase A has been used to produce homogeneous antibody drug conjugates (ADCs) with potencies that rival traditional ADCs used in the clinic 13.
Because wild-type Sortase A exhibits slow kinetics, BPS offers a modified sortase enzyme with improved kinetics (Sortase A Pentamutant, #71046). We also offer our convenient SiMPLe (Sortase Mediated Protein Ligation) protein labeling kit that includes all necessary buffers, purification materials and positive controls (SiMPLe Protein Labeling Kit, #79392), as well as the SiMPLe CHC Antibody Labeling Kit for site-specific antibody conjugation (#82155). Additionally, we offer evolved Sortase enzymes that exhibit more efficient antibody labeling at the N-terminus (Sortase A Hexamutant, #71047) and C-terminus (Sortase A Septamutant, #71048), respectively 14. Contact us today to learn more about the ways BPS Bioscience can provide the tools and expertise to help you fulfill your research goals.
References
[1] Guimaraes, C.P. et al. Identification of host cell factors required for intoxication through use of modified cholera toxin. J. Cell Biol. 195, 751-764 (2011).
[2] Gautier, A. et al. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 15, 128-136 (2008).
[3] Stephanopoulos, N. & Francis, M.B. Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol. 7, 876-884 (2011).
[4] Dirksen, A. & Dawson, P.E. Expanding the scope of chemoselective peptide ligation in chemical biology. Curr. Opin. Chem. Biol. 12, 760-766 (2008).
[5] Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, et al. Antibody–drug conjugates: current status and future directions. Drug Discovery Today 19, 869–81 (2014).
[6] Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102, 1458–65 (2003).
[7] Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Research 68(22):9280–90 (2008).
[8] Paterson, G.K. & Mitchell, T.J. The biology of Gram-positive sortase enzymes. Trends Microbiol. 12, 89-95 (2004).
[9] Ton-That, H., Liu, G., Mazmanian, S.K., Faull, K.F. & Schneewind, O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. USA 96, 12424-12429 (1999).
[10] Mazamanian, S.K., Liu, G., Ton-That, H. & Schneewind, O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285, 760-763 (1999).
[11] Spirig, T., Weiner, E.M. & Clubb, R.T. Sortase enzymes in Gram-positive bacteria. Mol. Microbiol. 82, 1044-1059 (2011).
[12] Ritzefeld, M. Sortagging: A robust and efficient chemoenzymatic ligation strategy. Chem. Eur. J. 20, 8516-8529 (2014).
[13] Beerli, R.R., Hell, T., Merkel, A.S. & Grawunder, U. Sortase enzyme-mediated generation of site-specifically conjugated antibody drug conjugates with High In Vitro and In Vivo Potency. PLoS One, 10 (2015).
[14]Chen, L., Cohen, J., Song, X., Zhao, A., Ye, Z., Feulner, C.J., Doonan, P., Somers, W., Lin, L. & Chen, P.R. Improved variants of SrtA for site-specific conjugation on antibodies and proteins with high efficiency. Sci. Rep. 6, 31899 (2016).r cells, Nat Immunol 9, 503-510.