MuRF3, SUMO-His-tags Recombinant
Catalog #
81054
$410
*
●
●
Purchase
Description
Human MuRF3 recombinant protein (Genbank Accession No. Q9BYV2), full length with N-terminal SUMO- and His-tags, expressed in E. coli.
●
Synonyms
E3 ubiquitin-protein ligase TRIM54, MURF3, MuRF-3, MURF-3, Muscle-specific RING finger protein 3, RING finger protein 30, RNF30, Tripartite motif-containing protein 54
●
Product Data Gallery
Product Info
Storage and Usage
Citations
Species
Human
Host Species/Expression System
E. coli
Purity
≥90% by SDS-PAGE
Format
Aqueous buffer solution
Formulation
50 mM Tris Buffer, pH 7.5, 150 mM NaCl, 10% glycerol, 5 mM DTT
MW
40.3 kDa (without SUMO tag)
Amino Acids
full length
Biological Activity
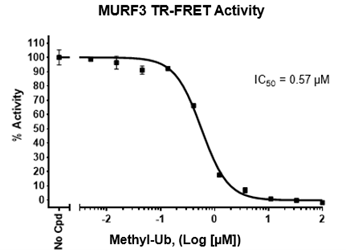
Typical enzyme concentration of 20-1000 nM is used for in vitro conjugation depending on assay conditions.
Genbank #
Q9BYV2
UniProt #
Q9BYV2
Background
MuRF3 is a RING domain E3 ligase that is involved in the conjugation of ubiquitin to target substrates. MuRF3 has been demonstrated to function with the E2 enzyme UBE2D3 (UbcH5c) in vitro. MuRF3 is also known as TRIM54 (tripartite motif containing 54) containing a RING-finger/B-box/coiled-coil tripartite fold. MuRF3 has been implicated along with MuRF1 as regulators of protein degradation in striated muscle. MuRF3 also plays important roles in maintaining cardiac function after myocardial infarction.
References
1. Balasubramanian, S., et al. Enhanced ubiquitination of cytoskeletal proteins in pressure overloaded myocardium is accompanied by changes in specific E3 ligases. J. Mol. Cell Cardiol., 2006. 41(4): p. 669-79.
2. Fielitz, J., et al. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J. Clin. Invest., 2007. 117(9): p. 2486-95.
3. Fielitz, J., et al. Loss of muscle-specific RING-finger 3 predisposes the heart to cardiac rupture after myocardial infarction. Proc. Natl. Acad. Sci. USA, 2007. 104(11): p. 4377-82.
2. Fielitz, J., et al. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J. Clin. Invest., 2007. 117(9): p. 2486-95.
3. Fielitz, J., et al. Loss of muscle-specific RING-finger 3 predisposes the heart to cardiac rupture after myocardial infarction. Proc. Natl. Acad. Sci. USA, 2007. 104(11): p. 4377-82.