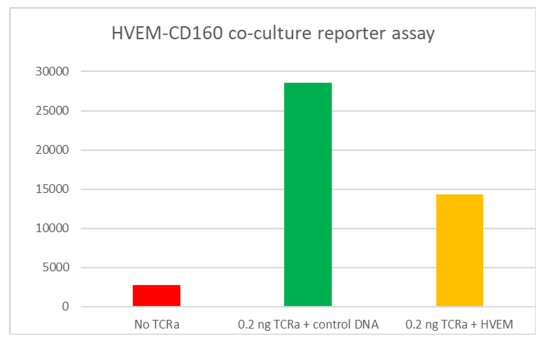
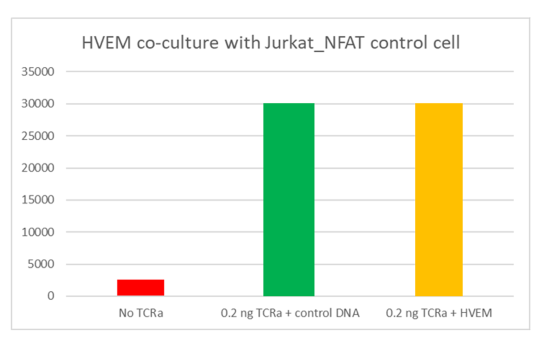
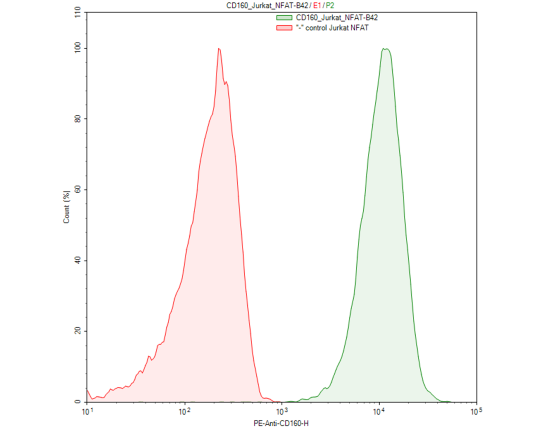
CD160/NFAT - Luciferase Reporter - Jurkat Recombinant Cell Line
Recombinant Jurkat T cell expressing firefly luciferase gene under the control of NFAT response elements with constitutive expression of human CD160. CD160 is a GPIanchored glycoprotein member of the Ig superfamily, also known as BY55, NK1, and NK28. GenBank Accession # NM_007053.
Interested in screening and profiling inhibitors, blocking antibodies, or activators of CD160 without the need to purchase and license the cell line? Check out our Cell Signaling Pathway Screening.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
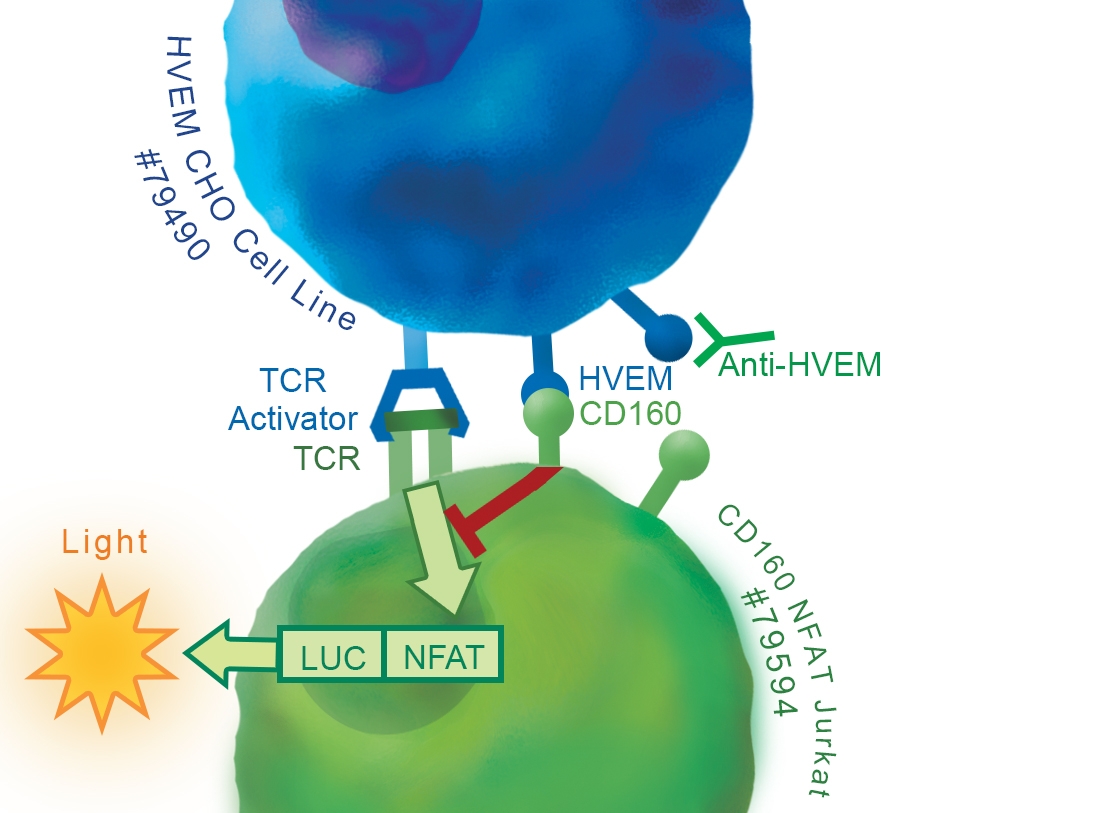
• CHO-K1 cell and its growth medium
• HVEM/CHO_TCR activator stable cell line (BPS Bioscience #79551)
• Transfection reagent for generating target cells (aka artificial Antigen Presenting Cells (aAPC)) [We use Lipofectamine™ 2000 (Life technologies #11668027). However, other transfection reagents work equally well.]
• Opti-MEM I Reduced Serum Medium (Life technologies #31985-062)
• Assay medium: Thaw Medium 2 (BPS Bioscience #60184)
• Anti-BTLA neutralizing antibody (for testing)
• Anti-HVEM neutralizing antibody (for testing)
• 96-well tissue culture-treated white clear-bottom assay plate
• One-Step luciferase assay system (BPS Bioscience #60690)
• Luminometer
CD160 is a glycosylphosphatidylinositol (GPI)-anchored protein member of the Ig superfamily that is expressed at the cell surface and highly restricted to circulating NK and T cells. Binding of CD160 to both classical and non-classical MHC I enhances NK and CD8+ CTL functions. However, engagement of CD160 by the Herpes Virus Entry Mediator (HVEM / TNFRSF14) was shown to mediate inhibition of CD4+ T-cell proliferation and TCR-mediated signaling. HVEM protein is a bimolecular switch that binds both co-stimulatory LT-α/LIGHT and co-inhibitory receptors CD160/BTLA. The binding of coinhibitory receptors CD160 and/or BTLA on T cells with HVEM expressed on DC or Tregs transduces negative signals into T cells that are counterbalanced by costimulatory signals delivered after direct engagement of HVEM on T cells by LIGHT expressed on DC or more likely, on other activated T cells (T–T cell cooperation).
HVEM was also shown to be expressed in the majority of cultured melanoma cell lines and metastatic melanoma samples. The predominance of the interaction of HVEM with CD160 and BTLA over the HVEM/LIGHT pathway or vice versa might be the result of differences in ligand/receptor affinity and the differential expression pattern of these molecules on cell types at different stages of cell differentiation. LIGHT, BTLA, and CD160 have substantially different binding affinities and occupy spatially distinct sites upon interaction with the HVEM receptor, which enables HVEM to function as a molecular switch.
The net effect of the LIGHT/HVEM and HVEM/BTLA/CD160 interaction, when these different receptors and ligands are simultaneously present, determines the outcome of the response. CD160/HVEM interaction plays a key role in the regulation of inflammatory, autoimmune, and antitumor responses, and is an important target for cancer immunotherapy drug discovery. The interaction of CD160 on tumor specific T cells and HVEM on melanoma cells resulted in T cell inhibition, which could be reversed by treatment with anti-CD160 blocking antibody. Therapeutically targeting CD160 and HVEM remains a focus for in pre-clinical studies as the bidirectional signaling pathways of CD160/BTLA/HVEM and HVEM/LIGHT are further elucidated.
1. del Rio, M.L., et al. HVEM/LIGHT/BTLA/CD160 co-signaling pathways as targets for immune regulation. Journal of Leukocyte Biology. 2010; 87(2): 223-35
2. Quan L. et al. BTLA marks a less cytotoxic T-cell subset in diffuse large B-cell lymphoma with high expression of checkpoints. Exp Hematol. 2018 Feb 3; 60: 47-56.
3. Torphy RJ, et al. Newly Emerging Immune Checkpoints: Promises for Future Cancer Therapy. Int J Mol Sci. 2017 Dec 6;18(12): E2642. Review.
4. Spodzieja M, et al. Design of short peptides to block BTLA/HVEM interactions for promoting anticancer T-cell responses. PLoS One. 2017 Jun 8;12(6): e0179201
5. Cara L Haymaker et al. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. OncoImmunology. August 2015; 4(8): e1014246.
6. Antonia SJ et al. Immunotherapy: Beyond Anti-PD-1 and Anti-PD-L1 Therapies. Am Soc Clin Oncol Educ Book. 2016; 35: e450-8
7. Zhang T, et al. Knockdown of HVEM, a Lymphocyte Regulator Gene, in Ovarian Cancer Cells Increases Sensitivity to Activated T Cells. Oncol Res. 2016; 24(3): 189-96
8. Lan X, et al. Increased BTLA and HVEM in gastric cancer are associated with progression and poor prognosis. Onco Targets Ther. 2017 Feb 16; 10: 919-926.
9. Zhao Q, et al. BTLA identifies dysfunctional PD-1-expressing CD4+ T cells in human hepatocellular carcinoma. Oncoimmunology. 2016 Nov 8; 5(12): e1254855.
10. Boice M, et al. Loss of the HVEM Tumor Suppressor in Lymphoma and Restoration by Modified CAR-T Cells. Cell. 2016 Oct 6; 167(2):405-418
11. Shui, J-W. and Kronenberg, M. HVEM is a TNF Receptor with Multiple Regulatory Roles in the Mucosal Immune System. Immune Network April, 2014; 14(2): 67-72.
12. Steinberg, M., et al. The Signaling Networks of the HVEM/BTLA in Immune Regulation. Immunol Rev. 2011 November; 244(1): 169–187