Histone Octamer (full length), His-tag Recombinant
Catalog #
52037
$480
*
●
●
Purchase
Description
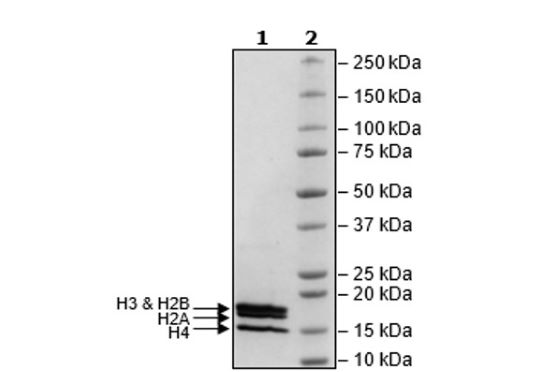
Human recombinant histone octamer consisting of 2 molecules each of histones H2A encompassing amino acids 2-130(end), H2B encompassing amino acids (2-126(end), H3 encompassing 2-136(end), and H4 encompassing amino acids 2-103(end). Each recombinant histone has an N-terminal His-tag (6xHis). The recombinant complex was affinity purified.
●
Product Data Gallery
Product Info
Storage and Usage
Citations
Species
Human
Construct
H2A (His-2-130(end))/ H2B (His-2-126(end))/ H3 (His-2-136(end))/ H4 (His-2-103(end))
Host Species/Expression System
E. coli
Purity
≥90
Format
Aqueous buffer solution
Formulation
45 mM Tris-HCl, pH 7.4, 2 M NaCl, and 10% glycerol
MW
H2A: 15 kDa; H2B: 15 kDa; H3: 16 kDa; and H4: 12 kDa
Amino Acids
H2A: 2-130(end); H2B: 2-126(end); H3: 2-136(end); H4: 2-103(end)
Genbank #
H2A: NM_033445; H2B: NM_003528; H3: NM_003532; H4: NM_003548
UniProt #
H2A: Q7L7L0; H2B: Q16778; H3: P68431; H4: P62805
Background
The histone octamer is composed of a central heterotetramer of two copies of both histones H3 and H4, flanked by two heterodimers of histones H2A and H2B. The octamer assembles when the H3/H4 tetramer complexes with two H2A/H2B dimers. These histones of the histone octamer all contain N-terminal tails that emanate from their central histone folds and are targets for epigenetic modification.
References
1. Miller KM, Jackson SP. Biochem Soc Trans 40(2):370-6 (2012).
2. Raut VV, Pandey SM, Sainis JK. Ann Bot 108(7):1235-46 (2011).
3. Park K, Fasman GD. Biochemistry. 1987 Dec 15;26(25):8042-5.
4. Meagher, R.B., Mussar, K.J., Epigenetics Chromatin 2012 Jul 20,5(1):11.