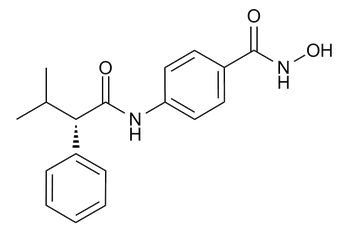
AR-42 (OSU-HDAC42)
Catalog #
27603-1
$195
*
●
●
Purchase
Description
AR-42 (also known as OSU-HDAC42), a derivative of hydroxamate-tethered phenylbutyrate is a broad-spectrum deacetylase inhibitor of both histone and non-histone proteins. AR-42 treatment induces histone hyperacetylation and p21WAF/CIP1 overexpression, inhibits gp130/Stat3 pathway and induces apoptosis and cell cycle arrest in multiple myeloma cells.
●
Synonyms
OSU-HDAC42; N-hydroxy-4-[[(2S)-3-methyl-2-phenylbutanoyl]amino]benzamide
●
Product Data Gallery
Product Info
Storage and Usage
Citations
Purity
≥99% by HPLC
Target(s)
HDAC
Formula
C18H20N2O3
MW
312.4 Da
Solubility
Soluble in DMSO
Biological Activity
AR-42 is a pan-HDAC inhibitor with IC50 of 16-30 nM. It has demonstrated greater potency and activity in solid tumors and hematological malignancies (including chronic lymphocyte leukemia, or CLL, B-cell lymphoma, prostate and ovarian cancers) when compared to vorinostat (SAHA), the first of two marketed compound in the class.
CAS Registry #
935881-37-1
Background
AR-42 induces histone H3 acetylation, α-tubulin acetylation and p21 up-regulation, which have been considered as the hallmark indicators of HDAC inhibition. AR-42 has been found to modulate several apoptosis inhibitors as well as cell survival regulator, including Akt, Bcl-xL, Bax, Ku70 and surviving, and exert potent antitumor activity against multiple tumor types, such as human prostate and hepatic cancers, at least partially through PI3K/Akt pathway inhibition. AR-42 has been designated an orphan drug by the FDA for the treatment of meningioma and schwannoma of the central nervous system. Meningioma and schwannoma are rare, benign tumors that can present in different locations within the brain and the spinal cord and may cause substantial morbidity.
References
1. Burns, S.S., et al. Cancer Res. 2013 Jan 15;73(2):792-803.
2. Zhang, S., et al. Int J Cancer. 2011 Jul 1;129(1):204-13.
3. Lucas, D.M., et al. PLoS One. 2010 Jun 3;5(6):e10941
2. Zhang, S., et al. Int J Cancer. 2011 Jul 1;129(1):204-13.
3. Lucas, D.M., et al. PLoS One. 2010 Jun 3;5(6):e10941