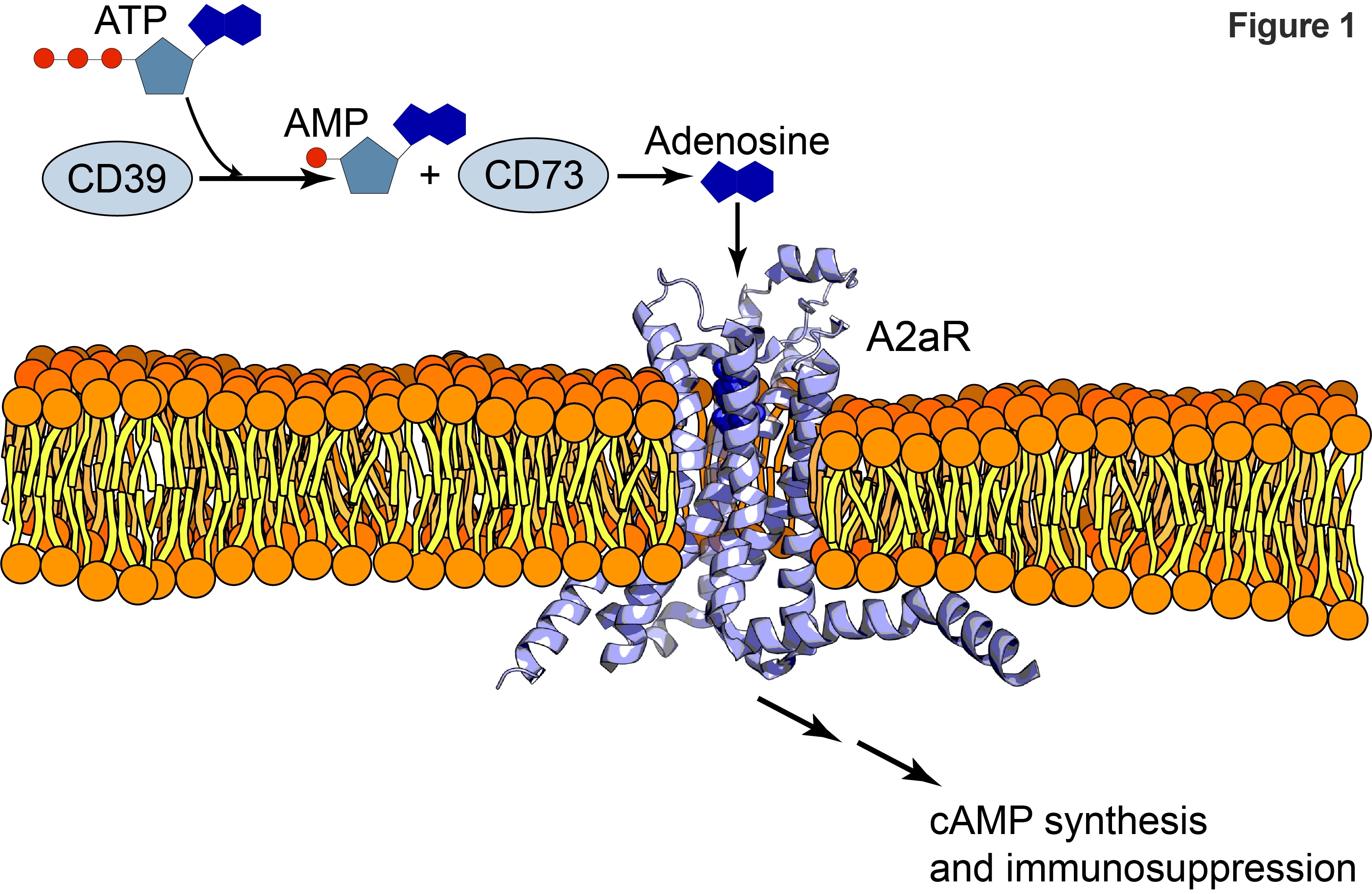
A2aR & Immunotherapy
The great need for treatments and the promise of cures are driving the search for second-generation immunotherapies beyond therapeutic antibodies targeting PD-1 and CTLA4 pathways. Immunotherapies are not limited to just therapeutic antibodies. Small-molecule inhibitors are showing great promise in clinical trials. For example, inhibitors targeting the enzymatic pathways, such as tryptophan catabolism are in late-phase clinical development. Preclinical and early clinical trials focused on immune regulation by extracellular ATP through the CD39-CD73-A2AR pathway combine the promise of treatment with therapeutic antibodies and the well-understood drug discovery techniques of small molecule inhibitors. Extra cellular ATP is a powerful stimulator and inhibitor of immunity in the tumor micro-environment. It produces pro-inflammatory signals by activating the NLRP3 inflamasome through interactions with receptors on Dendritic Cells 1. In contrast, it signals immune suppression through metabolism to adenosine via the CD39-CD73 pathway. The resulting adenosine stimulates the adenosine receptor (A2aR) triggering cAMP synthesis and leading to multiple, immunosuppressive pathways 2, 3. (See Figure 1)
A2aR is a G protein-coupled receptor expressed on a wide variety of cell types including many that regulate immune suppression. Stimulation on activated T-cells leads to inhibition of the NF-kB and MAPK pathways 4 . Ultimately this results in T-cell anergy. Similarly, activation of A2aR receptors on NK cells leads to reduced anti-tumor cytotoxicity (See Figure 2) 5. Activation on tumor-associated macrophages is also implicated in immuno-suppression. Deletion of A2aR on macrophages increases anti-tumor immunity through a T cell and NK cell dependent mechanism 6 .
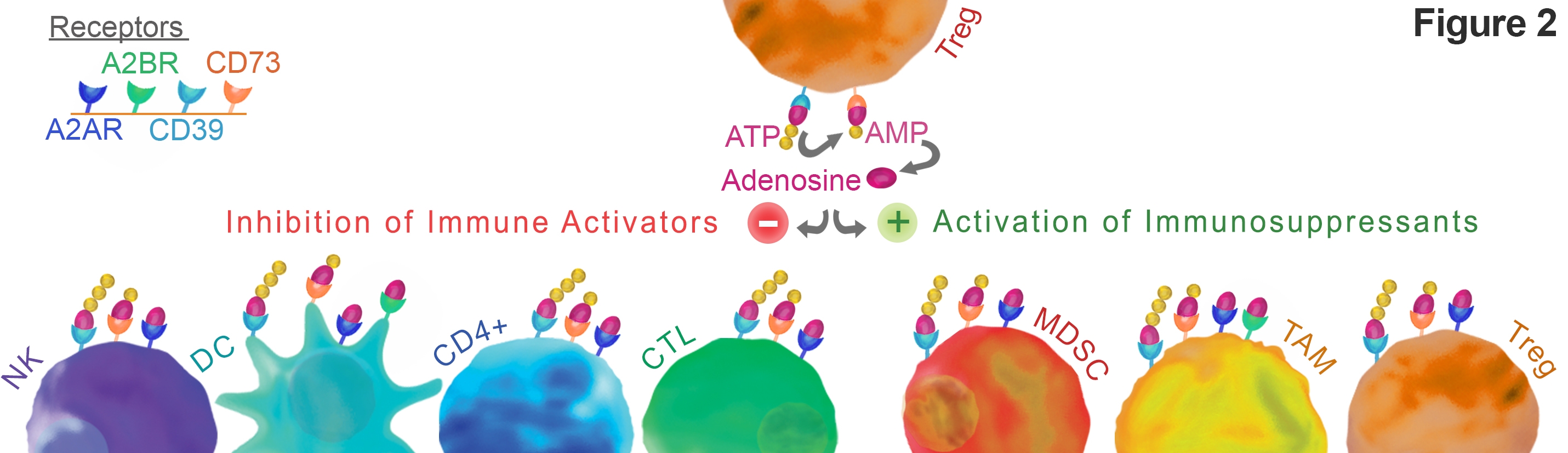
Rapid cell growth, death, and cellular stress in the TME tips the balance toward immunosuppression both by the increase of available ATP for metabolism and through the upregulation of CD39, CD73, and A2aR expression. The goal of tipping the balance back toward anti-tumor immunity has motivated investigation of A2aR as target for immunotherapy 2.
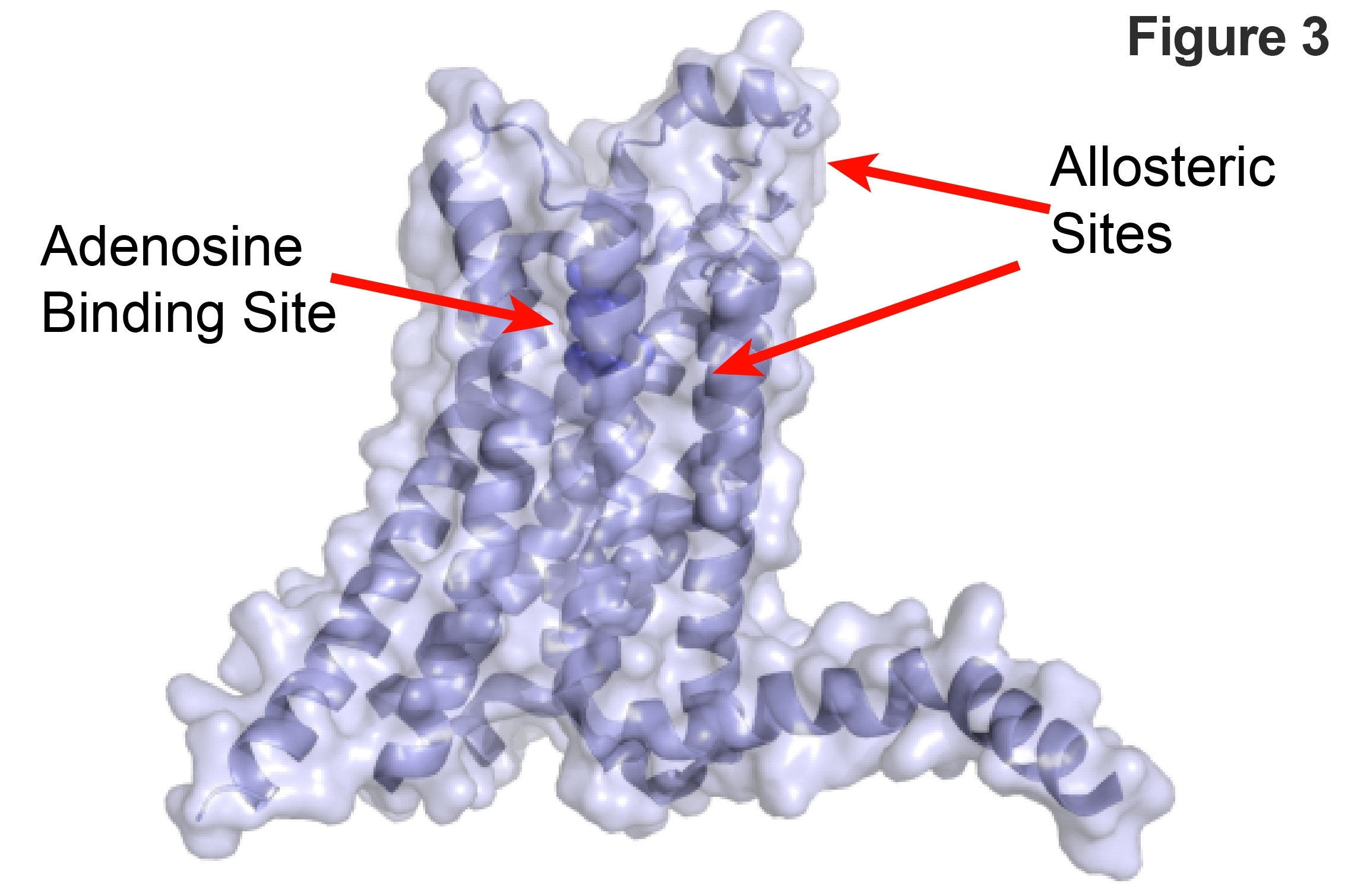
G-protein-coupled receptors are the most frequent target for small-molecule drug discovery 7 and there is a vast amount of biochemical and pharmacological data for driving research. Additionally a trove of structural data is now available for A2aR receptors in both inactive and active states 8, 9. The integration of this structural and functional data is providing new insights for structure based drug design including the identification of important allosteric inhibitors (See Figure 3). Identification of such allosteric sites are particularly interesting because they can lead to development of highly specific inhibitors.
The development of co-therapies based on inhibition of CD73 and A2aR is at the beginning of an exciting time. Recent experiments in double-knockout mice demonstrating synergy in loss of CD73 and A2aR on tumor inhibition suggest the benefit of targeting both molecules simultaneously 10. Furthermore, revelations on the engineering of effector functions into anti-CD73 therapeutic antibodies demonstrate the complex nature of co-therapy development for these targets 11.
Solving the puzzle of co-inhibition of A2aR and CD73 requires the proper research tools. BPS offers Adenosine A2aR stably expressed in HEK-293 reporter cells (Adenosine A2a Receptor Functional Cell Line #79381) and a control cell line with a cAMP-responsive luciferase construct (CRE/CREB Luc, #60515). This cellular tool complements our species-specific CD39 and CD73 proteins for both Human and Mouse models and our convenient CD39 (#79278) and CD73 Inhibitor Screening Assay Kits (#72058 & #72055).
References
[1] Ghiringhelli, F., Apetoh, L., Tesniere, A., Aymeric, L., Ma, Y., Ortiz, C., Vermaelen, K., Panaretakis, T., Mignot, G., Ullrich, E., Perfettini, J. L., Schlemmer, F., Tasdemir, E., Uhl, M., Genin, P., Civas, A., Ryffel, B., Kanellopoulos, J., Tschopp, J., Andre, F., Lidereau, R., McLaughlin, N. M., Haynes, N. M., Smyth, M. J., Kroemer, G., and Zitvogel, L. (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors, Nat Med 15, 1170-1178.
[2] Allard, B., Beavis, P. A., Darcy, P. K., and Stagg, J. (2016) Immunosuppressive activities of adenosine in cancer, Curr Opin Pharmacol 29, 7-16.
[3] Lebon, G., Warne, T., Edwards, P. C., Bennett, K., Langmead, C. J., Leslie, A. G., and Tate, C. G. (2011) Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation, Nature 474, 521-525.
[4] Romio, M., Reinbeck, B., Bongardt, S., Huls, S., Burghoff, S., and Schrader, J. (2011) Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells, Am J Physiol Cell Physiol 301, C530-539.
[5] Hausler, S. F., Del Barrio, I. M., Diessner, J., Stein, R. G., Strohschein, J., Honig, A., Dietl, J., and Wischhusen, J. (2014) Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion, Am J Transl Res 6, 129-139.
[6] Cekic, C., Day, Y. J., Sag, D., and Linden, J. (2014) Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment, Cancer Res 74, 7250-7259.
[7] Santos, R., Ursu, O., Gaulton, A., Bento, A. P., Donadi, R. S., Bologa, C. G., Karlsson, A., Al-Lazikani, B., Hersey, A., Oprea, T. I., and Overington, J. P. (2017) A comprehensive map of molecular drug targets, Nat Rev Drug Discov 16, 19-34.
[8] Ciancetta, A., and Jacobson, K. A. (2018) Breakthrough in GPCR Crystallography and Its Impact on Computer-Aided Drug Design, Methods Mol Biol 1705, 45-72.
[9] Jespers, W., Schiedel, A. C., Heitman, L. H., Cooke, R. M., Kleene, L., van Westen, G. J. P., Gloriam, D. E., Muller, C. E., Sotelo, E., and Gutierrez-de-Teran, H. (2018) Structural Mapping of Adenosine Receptor Mutations: Ligand Binding and Signaling Mechanisms, Trends Pharmacol Sci 39, 75-89.
[10] Young, A., Ngiow, S. F., Barkauskas, D. S., Sult, E., Hay, C., Blake, S. J., Huang, Q., Liu, J., Takeda, K., Teng, M. W., Sachsenmeier, K., and Smyth, M. J. (2016) Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses, Cancer Cell 30, 391-403.
[11] Dahan, R., and Ravetch, J. V. (2016) Co-targeting of Adenosine Signaling Pathways for Immunotherapy: Potentiation by Fc Receptor Engagement, Cancer Cell 30, 369-371.
Assay Data
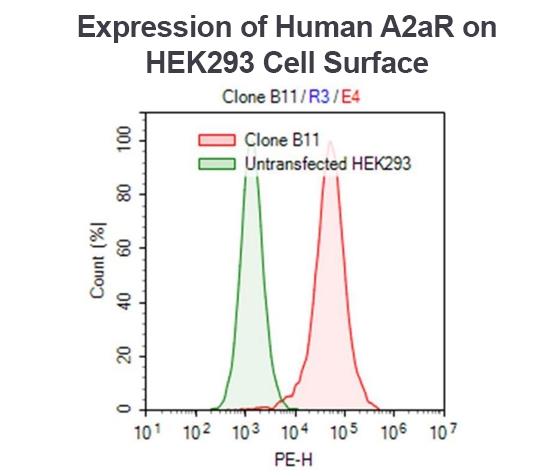
Using the A2aR Functional Cell Line (#79381), flow cytometry showed PE-conjugated anti-Human A2aR antibody detects A2aR-positive clonal population (clone B11) (red), using wild-type HEK293 cells as a negative control (green).
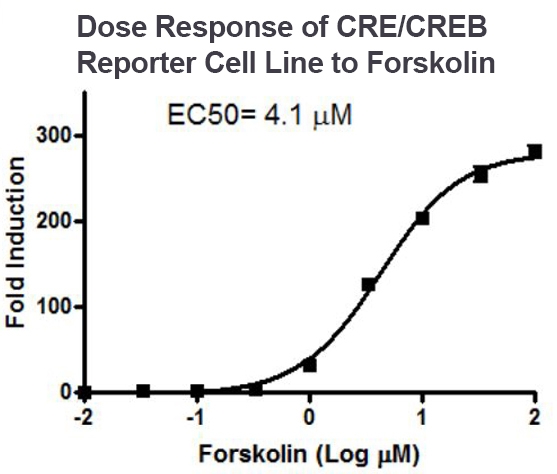
Fold induction of CRE/CREB luciferase reporter expression, as measured using the CRE/CREB Reporter (Luc) – HEK293 Recombinant Cell Line (#60515).
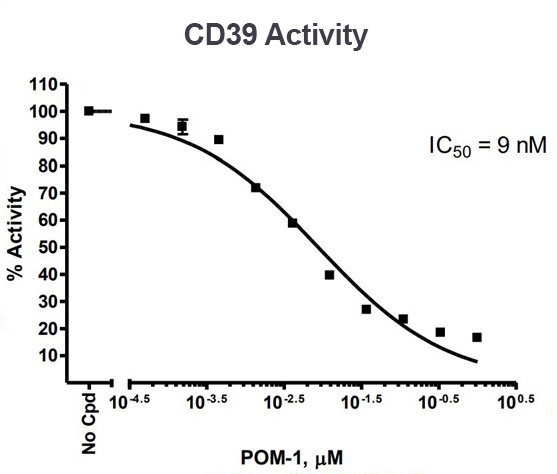
CD39 enzyme activity, measured using the CD39 Inhibitor Screening Assay Kit, Cat #79278
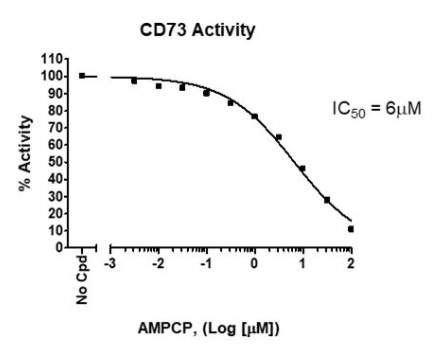
CD73 enzyme activity, measured using the CD73 Inhibitor Screening Assay Kit, Cat # 72058.
Proteins
Assay Kits
Services